Postpartum hemorrhage (PPH) remains a leading cause of maternal mortality worldwide. It is an emergent situation which can quickly escalate and then require invasive surgical intervention.
According to ACOG:
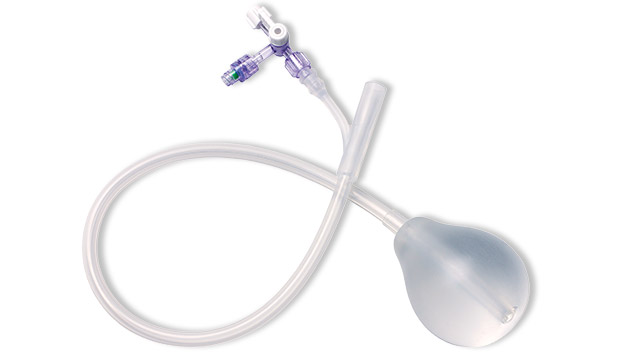
A systematic, stepwise approach to managing primary PPH includes the use of the BT-Cath® Balloon Tamponade Catheter. The advantages of using a balloon catheter for tamponade include timely utilization, ability to continuously monitor lost fluids, and less invasive removal procedure. The unique BT-Cath® features a soft silicone balloon that contours to the uterine wall. BT-Cath® is designed with check valves and is packaged with a bag-spike and two sterile syringes to aid in timely catheter utilization.
PRODUCT DESCRIPTION
BT-Cath® is a soft, silicone, dual lumen balloon tamponade catheter for managing postpartum hemorrhage (PPH).
FEATURES
INDICATIONS
BT-Cath® is intended to provide temporary control or reduction of uterine bleeding during postpartum hemorrhage that is unresponsive to standard therapy including massage and oxytocin administration.
CONTRAINDICATIONS
• Cervical cancer.
• Purulent infections in the vagina, cervix or uterus.
• Postpartum vaginal bleeding unaccompanied by uterine bleeding.
• Disseminated intravascular coagulation.
• Untreated uterine anomaly.
• Bleeding requiring surgical exploration (including hysterectomy) or angiographic embolization.
• Cases indicating hysterectomy.
• Pregnancy.
• A surgical site that would prohibit the device from effectively controlling the bleeding.
For more information, please contact us by EMAIL or call 1-800-533-4984 | 1-801-566-1200.
© Copyright 2024 Utah Medical Products, Inc. All rights reserved.